The test method for the core index of polyacrylamide
5.1 Determination of molecular weight
5.1.1 Method
Prepare dilute solution by adding the 85g/L sodium nitrate solution into the specimen and then determine its intrinsic viscosity with ubbelohde viscometer. The molecular weight of specimen shall be calculated according to empirical formula.
5.1.2 Reagent and solution
5.1.2.1 Sodium nitrate solution: 85 g/L。
5.1.3 Instrument and equipment
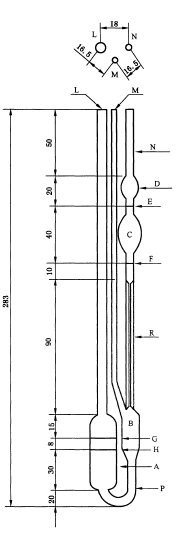
5.1.3.1 Ubbelohde viscometer (as shown in figure 1): capillary inner diameter 0.55 mm (±2%), At the temperature of 30℃±0.1℃, the time of 85 g/L sodium nitrate solution flowing through timing lines E and F is within 100s to 130s.
5. 1.3.2 Thermostatic waterbath: Can be controlled within 30℃±0.1℃.
5.1.3.3 Stopwatch: Division value is 0.1s.
5.1.3.4 Acid-resisting filter funnel: G3, 40 mL.
5.1.4 Analytical procedure
5.1.4.1 Determination of flowing-out time of sodium nitrate solution
Put the clean and dray ubbelohde viscometer into the thermostatic waterbath at 30℃±0.1℃ to keep the D ball completely submerged in the water. Add the sodium nitrate solution filtered with G3 acid-resisting filter funnel into ubbelohde viscometer until it reaches the level between filling lines G and H. Keep the temperature constant for 10 min to 15 min. Clamp the rubber tube of M pipe sleeve with a clamp. Use the rubber suction bulb to suck up the sodium nitrate solution into the D ball to fill half of it. Take down the rubber suction bulb and open M tube. Measure the time of sodium nitrate solution flowing trough of timing lines E and F. Repeat the determination three times and the errors should not exceed 0.2s. Adopt the mean value t0 of three measurements.
5.1.4.2 Preparation of test solution
Use the 50mL beaker with known mass to take about 0.03g solid specimen or colloid specimen with equivalent mass which shall be accurate to 0.2mg. After dissolved with sodium nitrate solution, the specimen shall be moved into the 100mL volumetric flask. Dissolve with sodium nitrate solution until the specimen reaches the required scale and then shake up.
5.1.4.3 Determination
Determine the time of flowing out of test solution by following the procedure of determining the flowing-out time of sodium nitrate solution described in 5.1.4.1.
5.1.5 Result calculation
Calculate the intrinsic viscosity [η] expressed in dL/g according to formula (1)
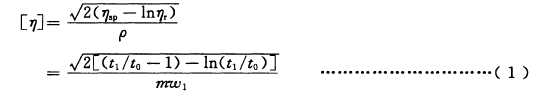
Where:
ηsp—Specific viscosity, 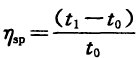 ;
;
ηr—Relative viscosity, 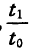
p—Mass concentration of test solution, g/dL;
t1—Time of flowing through the timing lines E and F on viscometer of test solution, s;
to—Time of flowing through the timing lines E and F on viscometer of sodium nitrate solution, s
m—Mass of specimen, g ;
w1—Mass fraction of solid content measured in 5.3, %.

expressed in millimeter (mm)
The molecular weight M should be calculated according to formula (2):

Where:
![]() —limit value of viscosity number, expressed in dL per g (dL/g);
—limit value of viscosity number, expressed in dL per g (dL/g);
K, a—empirical constants, their values should be chosen from Table 2 depending on different degree of hydrolysis.
Table 2
Degree of hydrolysis/% | K | a |
0 | 3.73×10-4 | 0.66 |
5 | 3.36×10-4 | 0.68 |
10 | 3.22×10-4 | 0.692 |
15 | 3.15×10-4 | 0.70 |
20 | 3.17×10-4 | 0.705 |
25 | 3.20×10-4 | 0.707 |
30 | 3.34×10-4 | 0.708 |
5.1.6 Tolerance
The arithmetic average of replicate results calculated separately will be taken as the final result. The relative difference of replicate results should be no greater than 5%.
5.2 Determination of degree of hydrolysis
5.2.1 Summary of method
Use the methyl-orange sodium indigotin-disulfonate as the indicator and titrate it with hydrochloric acid standard volumetric solution.
5.2.2 Reagent and material
5.2.2.1 Hydrochloric acid standard volumetric solution: c (HCl) approximately 0.1mol/L.
5.2.2.2 Methyl orange solution: lg/L.
5.2.2.3 Sodium indigotin-disulfonate solution: 2.5g/L, 10 days of service life.
5.2.3 Analytical procedure
Place a conical flask of 250 mL containing 100 mL of water onto the magnetic stirrer, and then put in a stirrer and start stirring. Measure off 0.03g of powder samples or equivalent colloidal specimens, accurate to 0.2 mg. Add the samples into the conical flasks and dissolve them completely. Add one drop of methyl orange and one drop of sodium indigotin-disulfonate and titrate them with hydrochloric acid standard volumetric solution, until the color of the solution changes from flavovirens to light gray.
5.2.4 Calculation of results
The degree of hydrolysis is determined based on mass fraction, and its value is expressed in % according to Formula (3):
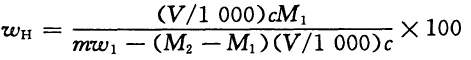 (3)
(3)
Where:
V—volume of hydrochloric acid standard volumetric solution consumed by titrating, expressed in milliliter (mL);
c—exact value of actual concentration of hydrochloric acid standard volumetric solution, expressed in mol per liter (mol/L);
M1—value of molar mass of acrylamide link, expressed in gram per mol (g/mol)(M1=71.07);
M2—value of molar mass of sodium acrylate, expressed in gram per mol (g/mol)(M2=94.04);
m—value of mass of specimen, expressed in gram (g);
w1—mass fraction of solid content of specimen, expressed in %.
5.2.5 Tolerance
The arithmetic average of replicate results calculated separately will be taken as the final result. The absolute difference of replicate results should be no greater than 1%.
5.3 Measurement of solid content
5.3.1 Summary of method
Dry the specimen in an electric drying oven at uniform temperature until its weight is constant.
5.3.2 Test instruments and equipment
General laboratory instruments and the following ones.
5.3.2.1 Electric drying oven: temperature can be stabilized to 120oC±2oC.
5.3.2.2 weighing bottle: Φ40 mm×30 mm or aluminum tray.
5.3.3 Analytical procedure
Measure off 1g (accurate to 0.2 mg) of specimen with a weighing bottle dried until its weight is constant at 120oC±2oC, and dry it in an electric drying oven at 120oC±2°C until its weight is constant.
5.3.4 Calculation of results
The solid content is determined based on mass fraction %, and its value is expressed in % according to Formula (4):
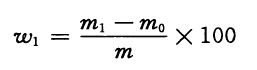 (4)
(4)
Where:
m1—value of masses of specimen and weighing bottle after drying, expressed in gram (g);
m0—value of masses of specimen and weighing bottle after drying, expressed in gram (g);
m—value of mass of specimen, expressed in gram (g).
5.3.5 Tolerance
The arithmetic average of replicate results calculated separately will be taken as the final result. The absolute difference of replicate results should be no greater than 0.5% for solid product and 0.3% for colloidal product.
5.4 Determination of content of acrylamide monomer
5.4.1 Gas chromatography (method for arbitration)
5.4.1.1 Summary of method
Leach the polyacrylamide to balance with the methanol-water solution of specified volume and concentration, and determine the area of chromatographic peak of acrylamide in leaching solution using gas chromatography method.
5.4.1.2 Reagent and material
5.4.1.2.1 Methanol.
5.4.1.2.2 Methanol-water mixed solvent: in volume ratio of 8:2.
5.4.1.2.3 Nitrogen gas: 99.99% in purity.
5.4.1.2.4 Carrier: Chromosorb W-HP, in grain size of 180µm-250µm.
5.4.1.2.5 Stationary liquid: polyethylene glycol, in relative molecular mass of 20000.
5.4.1.3 Test instruments and equipment
General laboratory instruments and the following ones.
5.4.1.3.1 Gas chromatograph: provided with flame ionization detector, with sensitivity less than or equal to l×10-10g/s.
5.4.1.3.2 Sample injector: 2µL or 5µLmicroinjector.
5.4.1.3.3 Chromatographic column: stainless steel column in length of 2m and in inner diameter of 3mm, with loading surface applied with ChromosorbW-HP carrier, polyethylene glycol stationary liquid in mass ratio of 20%. Before using, the chromatographic column should be aged at 175°C-180°C with nitrogen gas flowing at 20mL/min for over 12h.
5.4.1.3.4 Recorder: in full scale range of 5mV.
5.4.1.4 Preparation of test solution
5.4.1.4.1 Powdery polyacrylamide test solution
Measure off 2.9g-3.1g (accurate to 0.2 mg) of solid sample and add it into a 100mL dry plugged ground conical flask, and then measure off 30 mL of methanol-water mixed solvent into the conical flask, and put on the plug.
Shake the conical flask to allow the specimen scatter evenly, and place it at room temperature for 20h. Then fix the conical flask properly onto the KS oscillator, and ensure that the plug would not get loose, and shake it at room temperature for 4h. After standing, take the supernatant liquor as the solution sample.
Note: In addition to KS oscillator, the magnetic stirrer can also be used if only the specimen can be well mixed.
5.4.1.4.2 Colloidal polyacrylamide test solution
Measure off 9g-11g (accurate to 0.2 mg) of colloidal sample and add it into a 250mL dry plugged ground conical flask, and then add methanol in volume 4 times equivalent water content, and put on the plug. Follow the steps stated in 5.4.1.4.1.
5.4.1.5 Analytical procedure
5.4.1.5.1 Adjustment of instruments
Temperature of vaporizer: 230°C.
Column temperature: 165°C.
Temperature of detector: 230°C-240°C.
Gas flow rate: nitrogen gas 20mL/min; hydrogen gas 50mL/min; air 550mL/min.
Pressure before column: approximately 0.16MPa.
Chart speed of recorder: to be selectred according to requirements and width of chromatographic peak.
5.4.1.5.2 Calibration
5.4.1.5.2.1 External standard method: as per 5.15 of GB/T4946—1985.
5.4.1.5.2.2 Preparation of standard sample of acrylamide
Through secondary recrystallization treatment of industrial or chemical pure solid acrylamide, the standard sample in purity of 99% will be obtained.
5.4.1.5.2.3 Preparation of standard solution of acrylamide
Measure off 0.1000g±0.0001gacrylamide and add it into 100mL beaker, and then add about 15mL methanol-water mixed solvent to dissolve it. Then transfer them all to a 50 mL volumetric flask, and dilute it with methanol-water mixed solvent to graduation, and the standard solution in concentration of 2.00 mg/mL will be obtained.
Measure off 5mL and 10mL of standard solution of acrylamide in concentration of 2.00 mg/mL with pipetting into volumetric flask of 20mL, and dilute it with methanol-water mixed solvent until graduation, and the standard solution of acrylamide in concentration of 0.50mg/mL and 1.00mg/mL will be obtained.
Measure off 5mL of standard solution of acrylamide in concentration of 2.00 mg/mL with pipetting into volumetric flask of 50mL, and dilute it with methanol-water mixed solvent until graduation, and the standard solution of acrylamide in concentration of 0.20mg/mL will be obtained.
Measure off 1mL, 2mL, 5mL and 10mL of standard solution of acrylamide in concentration of 0.20 mg/mL with pipetting into 4 volumetric flasks of 20mL, and dilute them with methanol-water mixed solvent until graduation, and the standard solution of acrylamide in concentration of 0.01mg/mL, 0.02mg/mL, 0.05mg/mL and 0.1.0mg/mL will be obtained.
5.4.1.5.2.4 Drawing of calibration curve
Adjust the chromatograph according to 5.4.1.5.1 and allow it stabilize for some time. When the baseline of recorder becomes a straight line, measure off, with a microinjector, standard solutions of acrylamide in concentration of 0.01mg/mL, 0.02mg/mL, 0.05mg/mL, 0.10mg/mL, 0.20mg/mL, 0.50mg/mL, 1.00mg/mL and 2.00mg/mL respectively and inject them into the gas chromatograph, and then adjust it appropriately to all it to fade until it is at an appropriate position on the paper.
The areas will be calculated according to the size of chromatographic peak of standard solution of acrylamide recorded by the recorder.
The calibration curve will be drawn on the double logarithmic paper, with the content of various standard solutions of acrylamide as the x-axis, and with the areas of corresponding chromatographic peaks as the Y-axis. Two calibration curves can be drawn: one straight line can be obtained by connecting the areas of various chromatographic peaks corresponding to various points when the contents of acrylamide are equal to or less than 0.20mg/mL; and the other straight line can be obtained by connecting the areas of various chromatographic peaks corresponding to various points when the contents of acrylamide are above 0.10mg/mL.
5.4.1.5.3 Measurement
Under the conditions under 5.4.1.5.1, measure off 2µL of test solution and inject it into the gas chromatograph to obtain the corresponding chromatographic peak.
The areas will be calculated according to the size of chromatographic peak of standard solution of acrylamide in test solution recorded by the recorder.
The content of acrylamide will be found from the calibration curve according to the area of chromatographic peak.
5.4.1.6 Calculation of results
The acrylamide monomer is determined based on mass fraction w2, and its value is expressed in % according to Formula (5):
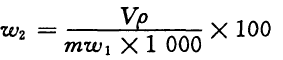 (5)
(5)
Where:
V—Total volume of methyl alcohol and water in test solution, mL;
p—Mass concentration of acrylamide found in calibration curve, mg/L;
m—Quality value of the test solution, g;
w1—Mass fraction of the solid content of test solution measured in 5.3, %.
5.4.1.7 Allowable deviation
Adopt the arithmetic average value of three parallel determination results as the measured result. The relative deviation of the single measured value and arithmetic average value shall to exceed 20%.
5.4.2 Bromination method
5.4.2.1 Method
Add excessive potassium bromate-potassium bromide solution into the test solution. In acidic medium, potassium bromate reacts with potassium bromide to form bromine, and then the formed bromine makes double bond reaction with acrylamide. After the reaction, add excessive potassium iodide to restore un-reacted bromine to form iodine, and then make re-titration of the precipitated iodine with sodium thiosulfate standard titration solution.
5. 4. 2. 2 Reagents and materials
5. 4. 2. 2. 1 Hydrochloric acid
5. 4. 2. 2. 2 Hydrochloric acid solution: 1+1
5. 4. 2. 2.3 Potassium iodide solution: 200g/L
5. 4. 2. 2. 4 Methyl alcohol- water extract: the volume ratio is 8:2
<span style="font-family: 'Times New Roman&a


